People are always confused about whether magnesium is a metal-like metal or a non-metal. The following periodic table shows that magnesium is an alkaline earth metal. So magnesium is also a metal in the periodic table. 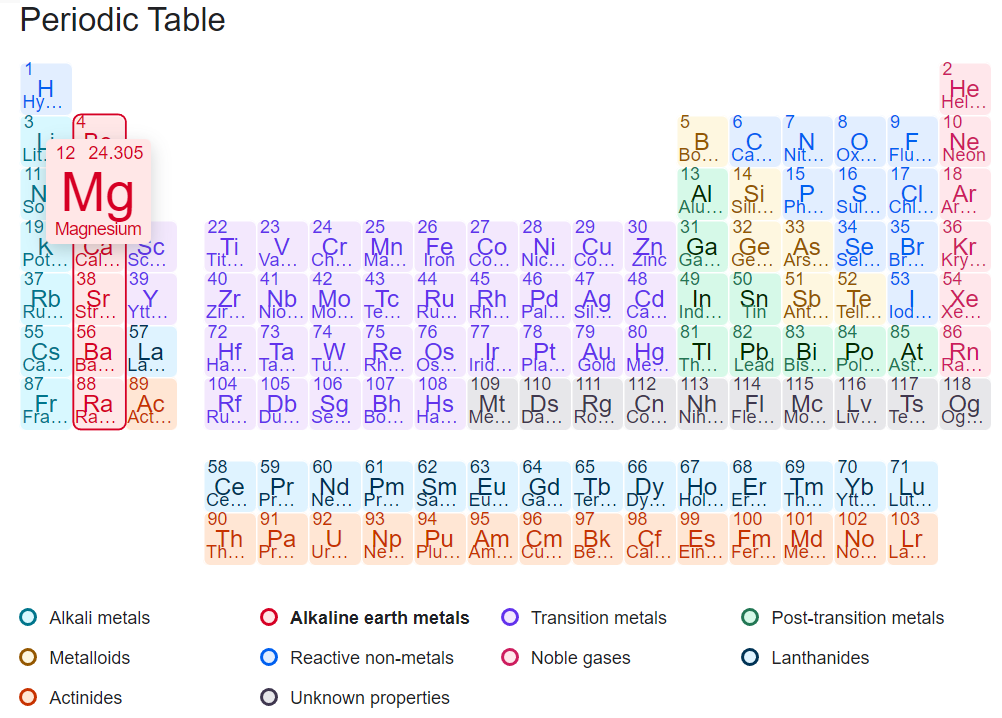
Table of Contents:
Metal elements are a group of chemical elements that have certain characteristics in common, including:
They are generally solid at room temperature (except for mercury).
They have a shiny, metallic appearance.
They are good conductors of heat and electricity.
They are malleable (able to be hammered or pressed into shape without breaking) and ductile (able to be drawn into wires).
They tend to have high melting and boiling points.
Magnesium is metal because it has properties that are characteristic of metals. We can provide magnesium alloy powder for you if you want.
Firstly, it is a good conductor of electricity and heat, which is a common property of metals. This is because metals have free electrons that can move easily through the material, allowing them to conduct electricity and heat.
Secondly, magnesium is malleable and ductile, meaning that it can be easily shaped into different forms without breaking. This property is also common in metals.
Lastly, magnesium has a metallic luster or shine, which is another characteristic of metals. This is because metals reflect light in a specific way due to the way their electrons interact with light waves.
Overall, these properties are what make magnesium metal, and it shares these properties with other metals like copper, iron, and aluminum. If you may have interest, we have magnesium metal block and other relative products.
Nonmetal elements are a group of chemical elements that do not have metallic properties. They are typically poor conductors of heat and electricity, have low melting and boiling points, and are generally not malleable or ductile. Nonmetal elements are found in various forms such as gases (e.g. oxygen, nitrogen), liquids (e.g. bromine), and solids (e.g. sulfur, carbon). They are located on the right-hand side of the periodic table and include elements such as hydrogen, carbon, nitrogen, oxygen, fluorine, chlorine, and others. Nonmetals play important roles in a variety of chemical and biological processes and are essential components of many molecules and compounds found in nature.
Metalloid elements, also known as semimetals, are a group of chemical elements that have properties of both metals and nonmetals. They have intermediate properties between those of metals and nonmetals and exhibit characteristics such as metallic luster, brittle texture, and semiconductor behavior. Metalloids are located on the periodic table between metals and nonmetals and include elements such as boron, silicon, germanium, arsenic, antimony, and tellurium. The properties of metalloids make them useful in a variety of applications, such as in the manufacture of computer chips, solar cells, and other electronic devices.
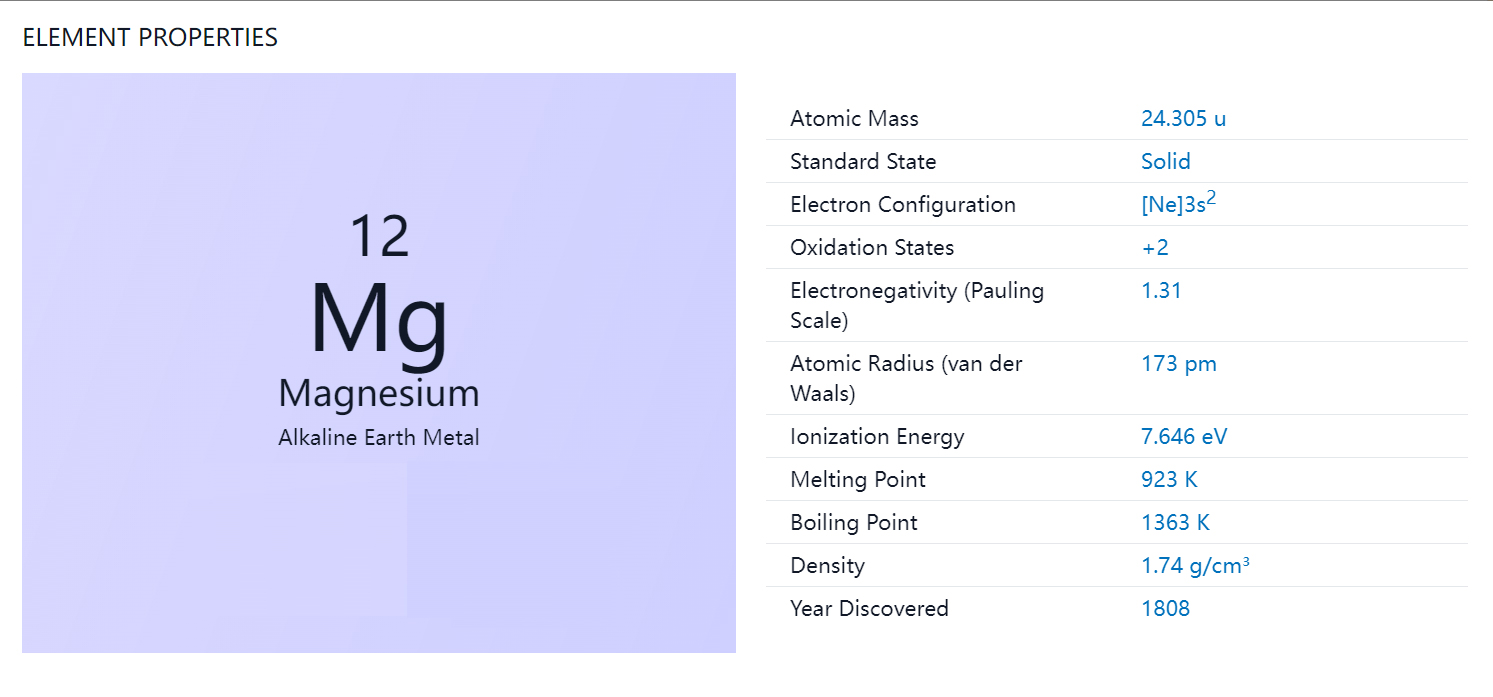
Magnesium metal is a chemical element with the symbol Mg and atomic number 12. It is a silver-white, lightweight metal that belongs to the alkaline earth metal group of elements. Magnesium is the eighth most abundant element in the Earth's crust and the fourth most common element in the Earth as a whole.
Magnesium metal has many uses due to its unique properties, such as its low density, high strength, and resistance to corrosion. It is used in the production of lightweight alloys for use in airplanes, cars, and other transportation vehicles. It is also used in the production of fireworks, flares, and other pyrotechnic devices due to its bright white flame. Magnesium also has medicinal uses, as it is an important mineral for the human body and is used as a laxative and antacid. And we can provide a reasonable magnesium alloy scrap price.
Yes, magnesium is considered a relatively soft metal. It has a low hardness and can be easily scratched or dented with a fingernail or other soft object. However, it is also a very lightweight metal with a high strength-to-weight ratio, which makes it useful for many applications.
Yes, magnesium is considered a strong metal, especially when its weight is taken into account. It has a high strength-to-weight ratio, which means that it can withstand a lot of force or pressure relative to its own weight. This property makes magnesium useful for many applications where strength and lightweight are important, such as in the aerospace and automotive industries. However, magnesium is not as strong as some other metals, such as steel or titanium, when compared in absolute terms.
No, magnesium is not considered a transition metal. Transition metals are elements that occupy the middle of the periodic table and have partially filled d orbitals in their electron configuration. Magnesium, on the other hand, is an alkaline earth metal that belongs to the s-block of elements. Its electron configuration is [Ne] 3s2, which means that it has two valence electrons in its outermost s orbital.
Yes, magnesium is considered a reactive metal. It readily reacts with many substances, including oxygen, water, acids, and other chemicals. When exposed to air, magnesium will react with the oxygen to form a thin layer of oxide on its surface, which can protect it from further reaction. However, this layer can also be disrupted, leading to rapid and sometimes violent reactions with other substances. For example, magnesium will react vigorously with acids to produce hydrogen gas and magnesium salts. Due to its reactivity, magnesium must be handled with care in order to avoid accidents.
Yes, magnesium is an alkaline earth metal. The alkaline earth metals are a group of elements in the periodic table that include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements are called "alkaline" because they form alkaline solutions when they react with water. Magnesium is the second element in this group and has many properties in common with the other alkaline earth metals, such as a low electron affinity, low electronegativity, and a tendency to form ionic compounds with nonmetals.
No, magnesium is not considered a heavy metal. Heavy metals are a group of elements that have a high atomic weight and density, such as lead, mercury, and cadmium. Magnesium, on the other hand, has a relatively low atomic weight and density, which is why it is often used in applications where a lightweight metal is needed. In fact, magnesium is one of the lightest structural metals available, which makes it useful for applications where weight is a critical factor, such as in the aerospace and automotive industries.